We've published a new paper
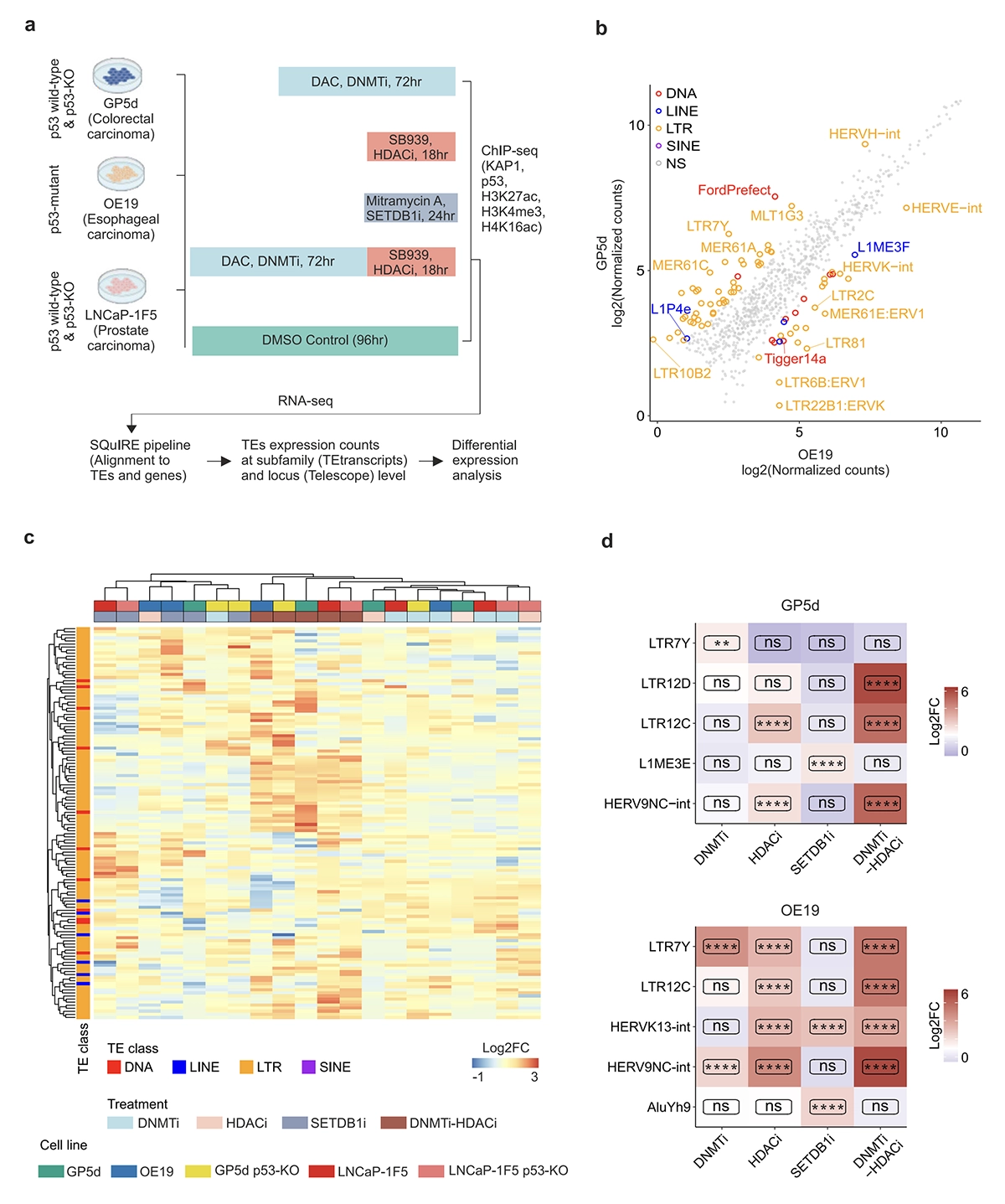
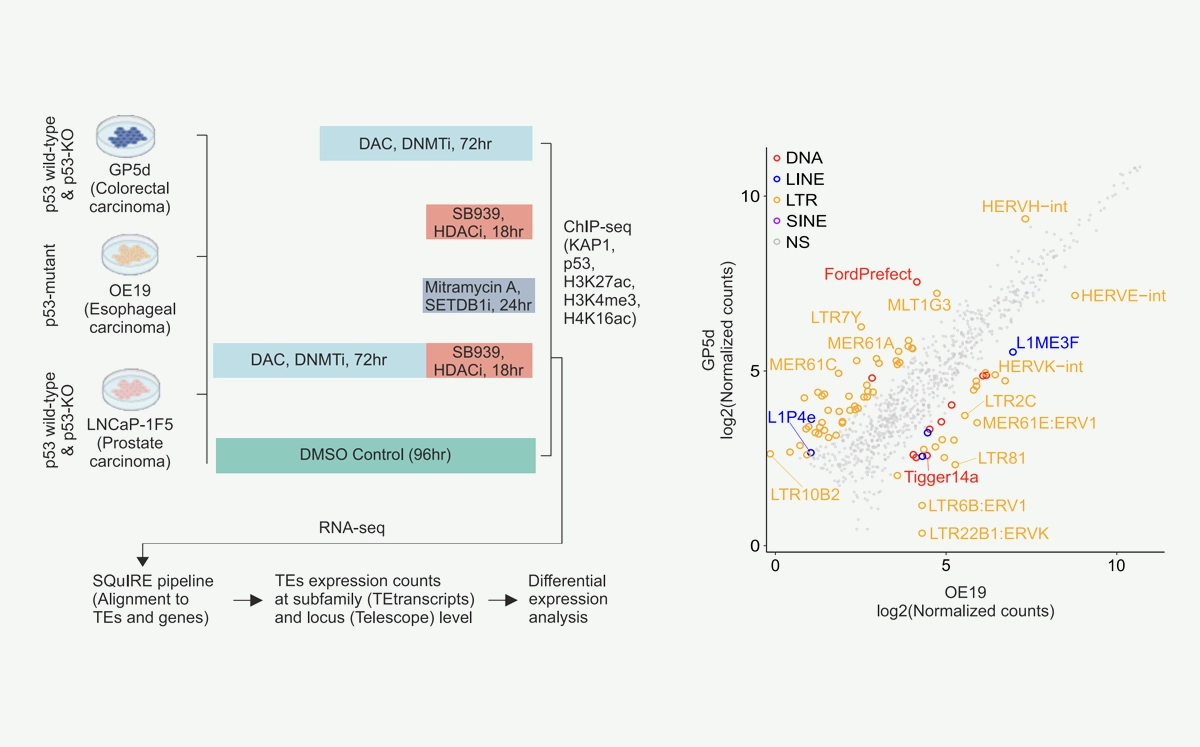
We’re proud to announce that our newest paper has been published in Communications Biology on 3rd July 2025, titled: Cancer cell type-specific derepression of transposable elements by inhibition of chromatin modifier enzymes.
Highlights
- Targeting chromatin-modifying enzymes leads to the activation of transposable elements, with both shared and cancer type-specific patterns
- Loss of p53 is associated with a stronger TE derepression and TE-chimeric transcript expression
- Co-inhibition of DNMT and HDAC induces accumulation of dsRNAs derived from inverted repeat Alu SINEs, accompanied by ADAR1 downregulation
- Combined epigenetic therapies simultaneously activate immunogenic TEs and suppress immune response inhibitors, offering a promising strategy to enhance cancer immunotherapy
Abstract
Derepression of transposable elements (TE) by epigenetic therapy leads to the activation of immune response in cancer cells. However, the molecular mechanism of TE regulation by distinct chromatin modifier enzymes (CME) in context of p53 is still elusive.
Here, we used FDA-approved epigenetic drugs to systematically inhibit distinct CMEs in p53 wild-type and p53-mutant colorectal, esophageal, and prostate cancer cells. We show that distinct TE subfamilies are derepressed by inhibition of different CMEs in cell type-specific manner. Co-inhibition of DNMT and HDAC (DNMTi-HDACi) had the most consistent effect across cancer types. Loss of p53 results in stronger TE activation and TE-chimeric transcript expression and this effect is largely mediated by the non-genomic actions of p53. Robust immune response elicited by DNMTi-HDACi is due to induced inverted repeat Alu expression concomitant with reduced ADAR1-mediated Alu RNA editing.
Collectively, our systematic analyses provide insights for rational use of epigenetic therapies in distinct cancers.